Mineral type: Zeolite
Clinoptilolite is a zeolite, meaning it is characterized by its unique microporous, three-dimensional crystalline structure made of aluminosilicate.
While both zeolites and clays are aluminosilicate minerals, zeolites are distinguished by their porous, three-dimensional microporous network structure with interconnected cavities and channels, unlike the layered structure of clays which allows for swelling and shrinking in water. Clinoptilolite can absorb and desorb H20 indefinitely without changing its form.
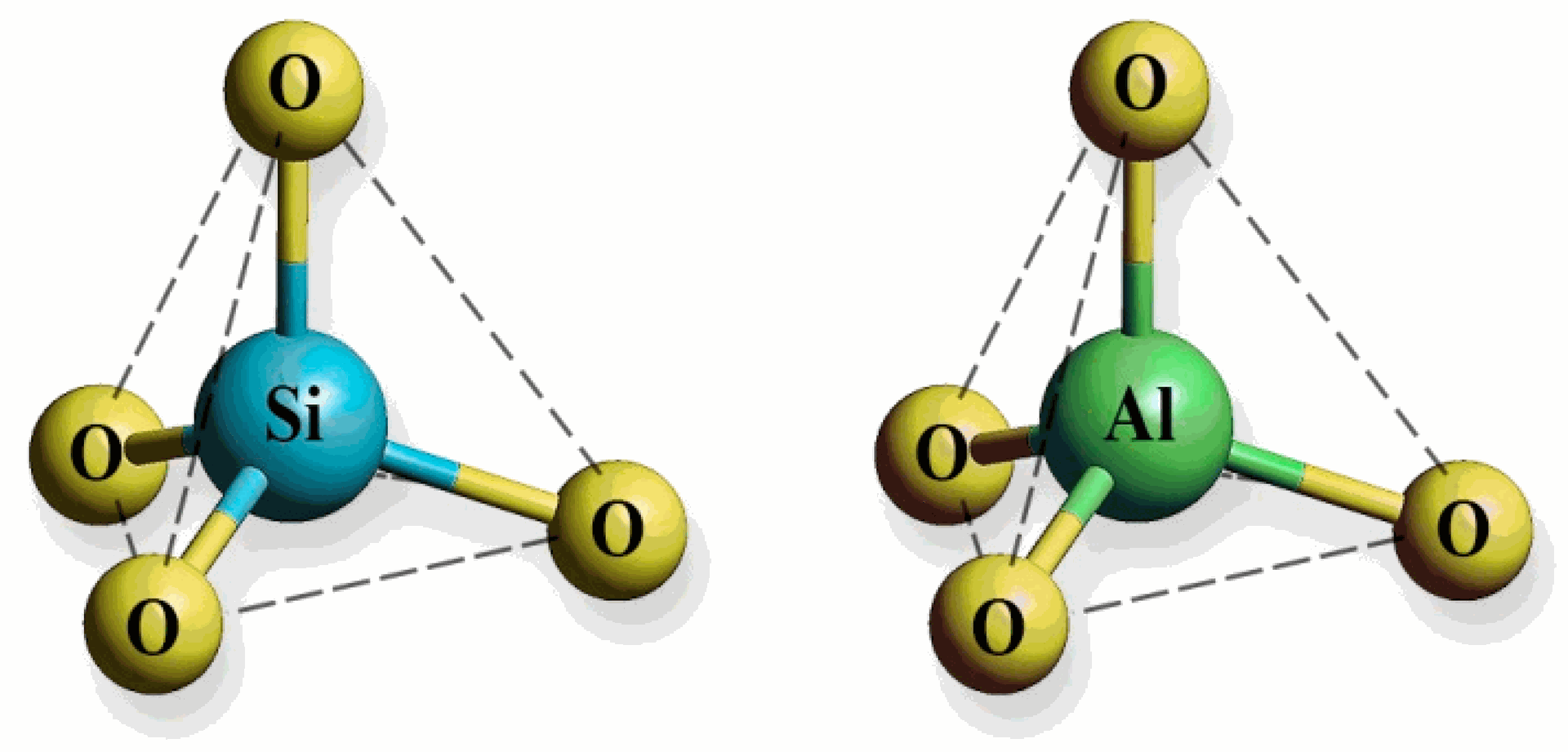
Figure 1. Molecular geometry of a silicate (SiO4) tetrahedron and an aluminate (AlO4) tetrahedron. Both structures are tetrahedral, with a central silicon or aluminum atom bonded to four oxygen atoms.
Clinoptilolite is a heulandite-type zeolite mineral that is a nontoxic microporous hydrated aluminosilicate. It is characterized by a stable 3-D framework of linked SiO4 and AlO4 tetrahedra, with intervening open channels and cages. Partial substitution of Si4+ by Al3+ results in an overall negatively charged crystal lattice, largely neutralized with diverse exchangeable extra framework cations (commonly K+, Na+, Ca2+, and/or Mg2+) that are loosely bound within the open pore structure. Water molecules also occupy open framework cavities.
![Figure 1. Clinoptilolite structure (generated by VESTA [3])](/web/image/1163-87327d94/crystals-14-00646-g001.png)
Figure 2. Clinoptilolite structure (generated by VESTA [3])





